Registration
Registration fees for ICCBH 2024 are as follows:
When you are ready to register click on the ‘Register here’ button, this will take you through to the registration system. When you click on ‘Buy tickets’ you will be asked to create your own personal log in and password (if you have already submitted an abstract you can use the same log in details).
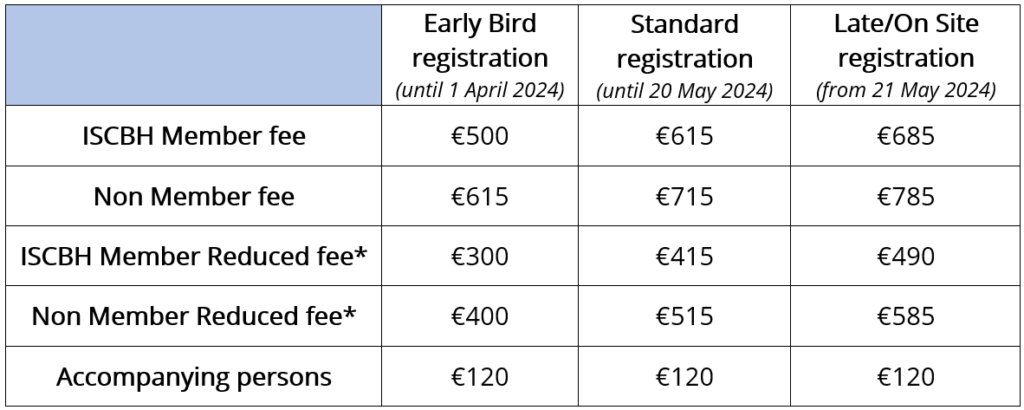
*Reduced fee can be paid by students (PhD students, undergraduates), Junior Post Docs (within 5 years of PhD), residents, fellows, Allied Health Professionals, patient organisation representatives, delegates based in a low or developing income country (World Bank criteria) and retirees.
Accompanying persons: should you be travelling with a partner you can purchase a ticket for them to join the social events. This does not give them access to the scientific sessions or the exhibition hall during the day.
Included in the delegate registration fee:
- All scientific sessions
- Pre-meeting morning sessions/workshops on Saturday 22 June
- Commerical Exhibition
- Printed Programme Book
- All refreshment breaks, including lunch (as per programme)
- Welcome Reception Ticket – Saturday 22 June
There is a nominal charge of €60.00 per ticket for the Conference Dinner which will take place on Monday 24 June 2024. Tickets can be purchased when you register or you can return to your registration at a later date to add a ticket should you wish to. These will be subject to availability.
Included in the accompanying person fee:
- Welcome Reception – Saturday 22 June
- Conference Dinner – Monday 24 June
For reduced fee bookings you will be required to complete an Eligibility form which you can download HERE – this form not required for low-middle income countries.
Single Day Registrations:
We do offer single day registrations, if you are interested in more information about these, please contact the ICCBH Events team on events@theiscbh.org
The meeting programme is designed for healthcare professionals and researchers. Pharmaceutical industry regulations do not allow open invitations to members of the public to attend. However, patient organisation representatives may register to attend the meeting provided they meet the following criteria (evidence in the form of a letter on the organisation’s letterhead is required):
- They represent a formally registered international organisation with elections (or national with some international activities) and
- They are a member of the management group or are officially nominated by the management group or are a paid member of staff
Children under 16 years of age will not be allowed access into the exhibition hall.
Information for Delegates – more detailed information for our attending delegates will be added here nearer the time.
Information for Presenters – more detailed information for those presenting, either faculty talks, oral communications or for printed posters can be found below:
Dates to remember:
15 Nov 2023 – Abstract submission open
30 Nov 2023 – Delegate registration open
5 Feb 2024 – Deadline for abstracts
11 Mar 2024 – Confirmations to authors
1 Apr 2024 – Deadline for early bird registration
8 Apr 2024 – Late breaking abstract submission opens
6 May 2024 – Deadline for late breaking abstracts
20 May 2024 – Confirmation to authors of late breaking abstracts
20 May 2024 – Deadline for standard registrations (late fee applies from this date)
Confirmation of your registration will only be sent when payment has been received. Payment must be received by the relevant deadlines in order to qualify for the discounted fees.
Early Bird Registration Deadline: 1 April 2024, 23:59 CEST
Standard Registration Deadline: 20 May 2024, 23:59 CEST
(after the pre-registration deadline on-site fees will apply)
There are options for delegates to book accommodation themselves at a selection of hotels – more information can be found on the accommodation page of the website.
In case you have any queries about your registration or accommodation please contact the ICCBH Conference team via email events:theiscbh.org
Group bookings
Please contact the ICCBH Events Team by email events@theiscbh.org to arrange bookings for groups of 10 or more.
Photography, Audio Recording and Video Recording
Photography, audio recording and video recording will be taking place at the conference. The release, publication, exhibition or reproduction of this content may be used for newsletters, webcasts, promotional purposes, advertising, inclusion on websites, social media or any other purposes by the ISCBH. Please notify us if you wish to be removed from any such materials in advance of the conference by emailing the Events team at events@theiscbh.org. Alternatively, if you do not agree, please let the photographer know at the conference.
You are respectfully requested not to take photos or recordings during the scientific sessions.
Code of Conduct
This conference is dedicated to providing a harassment-free experience for everyone, regardless of gender, age, sexual orientation, disability, physical appearance, body size, ethnicity, religion (or lack thereof), or technology choices. We do not tolerate harassment of our delegates in any form. Inappropriate language and imagery are not acceptable to be included in presentations, workshops, networking forums and other online media. Conference delegates violating these rules may be sanctioned or expelled from the conference without refund at the discretion of the conference organisers.
Data Consent
By registering for this conference, delegates agree to the collection of contact and demographic information. This includes any information unique to the delegate that is available on the individual’s profile. The organisers will share limited information (name, title, organisation and country only) in the official delegate list.
Italian Pharmaceutical Companies – Official Italian Agency
Any Italian pharmaceutical company supporting or participating in a congress abroad, is subjected to an authorization by AIFA (Italian Drug Agency), according to an Italian Government Decree (Decreto Legislativo 219/06 – art. 124).
The authorisation request must be submitted within 70 days before the start date of the event. The appointed agency to collect all applications from pharmaceutical companies and file them with the AIFA is:
Fargo International
Via P. Maroncelli 50
50137 FIRENZE ITALY
Mob. + 39 3203830442 Benedetta
Mob. +39 3335202862 Giulia
Email: b.cambria@alifargoint.it
TERMS & CONDITIONS
Payment Policy
Payment can only be accepted by Visa or Mastercard. Payment by credit card will be charged straight away. Once payment goes through you will receive a copy of the invoice and receipt in your confirmation email.
Cancellation & Refund Policy
If cancelled on or before 11 March 2024 – 100% of the registration fee is refundable
If cancelled from 12 March to 8 April 2024 – 50% of the registration fee is refundable
No refunds are payable from 9 April 2024 onwards
Please note if you pre-register and do not attend the conference you will be charged your participation fee and will be required to settle any outstanding balances.
In the unlikely case of cancellation of the event, the organisers shall not accept liability for any consequential loss and shall have no liability to reimburse any other costs that mat have been incurred, including transport costs, accommodation etc. Delegates are encouraged to take out travel insurance when making travel and accommodation arrangements.
Changes & Substitutions of Registrations
Substitutions/replacements can be made at any time and are subject to a €25 + VAT administration fee. Notification of changes or cancellation must be made in writing and sent to the ICCBH Events tean at events@theiscbh.org
Insurance, release and waiver of liability
It is highly recommended that all participants carry appropriate health and travel insurance. The organisers cannot accept liability for individual issues relating to medical, travel or personal insurance.


